Abstract
Introduction
Current DLBCL prognostic scores are poor predictors of individual risk with 26% of "very good" and "good" risk patients having unfavourable outcomes. Hypercalcaemia has been associated with poor outcomes in several cancers including multiple myeloma, but is not included in current DLBCL prognostic scoring models.
Aim
To determine the prognostic significance of hypercalcaemia in newly diagnosed DLBCL and identify relationships between hypercalcaemia and other established prognostic variables including cell of origin, and the components of the revised international prognostic index (R-IPI including impaired performance status, older age, advanced stage, elevated lactate dehydrogenase, and extranodal involvement.
Methods
Retrospective cohort study at two academic healthcare networks in Melbourne, Australia. All patients with a newly diagnosed DLBCL by WHO 2008 criteria were eligible for inclusion.
Cases were identified from hospital lymphoma databases. Only cases with adequate clinical information including baseline characteristics, therapy received and outcomes were included. Cell of origin was determined by the modified Hans criteria. At Monash Health, patients were included from September 2002 until December 2015, and from Western Health, patients were included from January 2012 until December 2015. Hypercalcaemia was defined as a serum calcium >2.6mmol/L within 30 days prior to, or 15 days following the diagnosis of DLBCL. Serum calcium levels were corrected for hypoalbuminaemia if present. Immunohistochemical cell of origin was recorded using modified Hans criteria (Meyer et al. 2011). Survival correlates were estimated by Kaplan-Meier log rank method. Multivariable analysis was performed by Cox regression. Relationships between serum calcium and other covariates were examined by t test for continuous variables and χ2 for categorical variables.
This project was approved by the human research review boards at each institution.
Results
A total of 514 patients were identified as being eligible for inclusion, of which 41 (7.9%) were identified to have calcium >2.6mmol/L. No patients had an alternate endocrine cause of hypercalcaemia identified.
Patients with hypercalcaemia had higher median age (75.3 vs. 66.2 years, P <0.001), and higher proportion with poor R-IPI (80% vs. 46%, P <0.001), advanced stage (78.0% vs. 57.5%, P=0.010), elevated LDH (87.8% vs. 52.2%, P <0.001) and non-GCB cell of origin (53.3% vs. 36.6%, P=0.049).
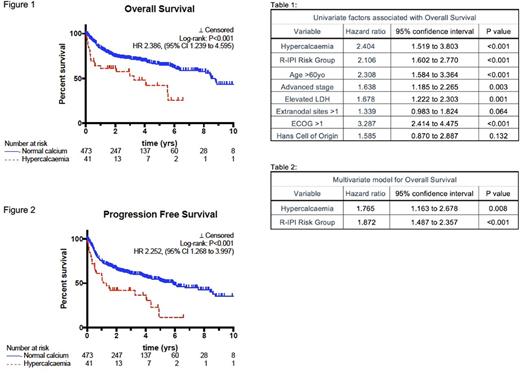
Excluding patients treated with palliative intent, patients with hypercalcaemia had worse clinical outcomes with shorter median OS (3.3 vs. 8.8 years, P <0.001 HR 2.34, 95% CI 1.24 to 4.60, see figure 1) and median PFS (1.3yrs vs. 5.9 years, P <0.001, HR 2.25, 95%CI 1.27 to 4.00, see figure 2). The univariate adverse impact of hypercalcaemia on OS (HR 2.404, 95% CI: 1.519 to 3.803, P<0.001, see table 1) and PFS (HR 2.267, 95% CI: 1.504 to 3.419, P<0.001) remained independent of R-IPI on multivariate analysis (see table 2).
Conclusion
Hypercalcaemia is an independent adverse prognostic factor and should be considered for incorporation into future risk scores for DLBCL. The mechanisms of hypercalcaemia and its association with non-GCB type DLBCL warrants further investigation.
Fedele: Celgene: Other: Travel grant to attend ASH. Low: Merck: Honoraria. Opat: Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Research Funding; Mundipharma: Consultancy; Beigene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal